Researchers have recently unveiled a new experimental drug called “Baxdrostat,” produced by AstraZeneca, which has shown promising results in treating cases of high blood pressure that are difficult to control even with traditional medications. If approved by regulatory authorities, it will be one of the first innovative solutions for treating high blood pressure in several decades.
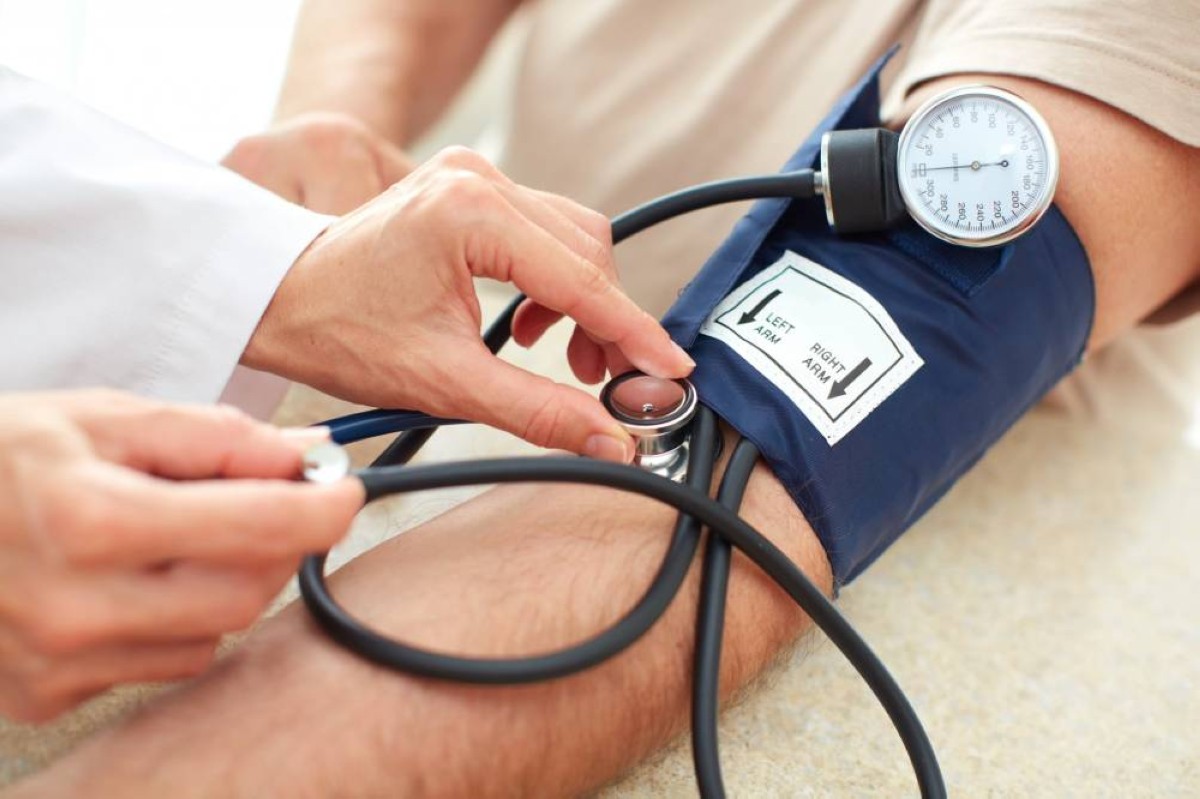
The clinical trial results, involving 800 patients with high blood pressure despite taking two or more medications for at least four weeks, were presented at the European Society of Cardiology conference in Madrid and simultaneously published in the New England Journal of Medicine. The trial required participants to have systolic blood pressure between 140 and 170 mmHg.
Participants were divided into three groups: the first received a 1 mg dose of Baxdrostat, the second 2 mg, and the third a placebo. After 12 weeks, the study recorded that 40% of those who took Baxdrostat reached normal blood pressure levels, compared to less than 20% in the placebo group. Systolic pressure readings dropped by 9-10 mmHg more in the treatment groups than in the placebo group, a decrease sufficient to reduce the risk of heart diseases according to studies.
High blood pressure increases the burden on the heart and blood vessels, making it difficult for blood to reach vital organs, raising the risk of heart disease, stroke, vascular dementia, and cognitive problems. Lowering blood pressure is the most effective way to avoid these deadly complications, especially since heart diseases remain the leading cause of death worldwide.
Half of adults in the United States suffer from high blood pressure, and 1 in 10 faces so-called resistant hypertension, meaning it does not respond to three or more medications. Adding Baxdrostat will provide an important new option, according to Dr. Stacey E. Rosen, president of the American Heart Association.
Currently available drugs work through different mechanisms: dilating blood vessels, increasing urine output to eliminate salt and fluids, affecting the nervous system, reducing the secretion of the hormone “angiotensin,” or decreasing the body’s retention of water and salt. Each can be associated with various side effects, including dizziness, fatigue, leg swelling, and heart rhythm disturbances.
The study showed that Baxdrostat’s side effects were generally mild, most commonly disturbances in potassium and sodium levels, but these were rare. Baxdrostat works by inhibiting the hormone “aldosterone” secreted by the adrenal glands, which regulates salt and water in the body. In cases of excess aldosterone production, the body retains large amounts of fluids and salts, leading to high blood pressure.
Dr. Jennifer Brown, co-author of the study, explains that many heart patients need strong and rapid blood pressure control to avoid the possibility of another heart attack, and some cannot tolerate traditional medications or do not get the desired effect. Therefore, Baxdrostat may be an effective complementary option in these cases.
Observers from the Universities of Edinburgh and Manchester confirmed in an accompanying scientific publication that further studies are needed to identify the patients best suited for this treatment and to provide long-term data to determine its long-term effectiveness. If proven, it could become the cornerstone treatment for stubborn high blood pressure cases.
AstraZeneca is scheduled to submit its data to regulatory authorities by the end of 2025.











Recommended for you
Talib Al-Rifai Chronicles Kuwaiti Art Heritage in "Doukhi.. Tasaseem Al-Saba"
Exhibition City Completes About 80% of Preparations for the Damascus International Fair Launch
Unified Admission Applications Start Tuesday with 640 Students to be Accepted in Medicine
Egypt Post: We Have Over 10 Million Customers in Savings Accounts and Offer Daily, Monthly, and Annual Returns
Al-Jaghbeer: The Industrial Sector Leads Economic Growth
His Highness Sheikh Isa bin Salman bin Hamad Al Khalifa Receives the United States Ambassador to the Kingdom of Bahrain